Persistent Effects of Historical Selective Logging on a Vascular Epiphyte Assemblage in the Forest Canopy of the Western Ghats, India
- 1Centre for Ecological Sciences, Indian Institute of Science, Bengaluru, India
- 2Ashoka Trust for Research in Ecology and the Environment, Bengaluru, India
Forest canopies have been dubbed the last biological frontier and continue to remain underexplored. Vascular epiphytes form a rich assemblage of plants within the forest canopy and apart from sustaining diverse taxa, they also fulfill critical ecological functions. Vascular epiphytes are particularly sensitive to perturbations of microclimate and microhabitat within the canopy, especially from anthropogenic changes such as logging. The forests of the megadiverse Western Ghats in India harbor a rich assemblage of vascular epiphytes, but their ecology has not been examined systematically. We compared the diversity, abundance, and composition of a vascular epiphyte assemblage between an unlogged and a historically selectively logged forest in the southern Western Ghats, India, and identified factors affecting the epiphyte assemblage. Canopies of 100 trees each in selectively logged and unlogged forests were accessed using the single-rope technique. We found 20 species of vascular epiphytes with the assemblage dominated by members of Orchidaceae. The diversity and abundance of epiphytes were significantly greater in the selectively logged forest. One host tree, Cullenia exarillata, supported the greatest number of epiphytes in both forest stands. The niche widths of epiphyte species, computed with host tree species as a resource, were similar between the two stands but a greater number of species pairs overlapped in the selectively logged forest. Overall, epiphyte abundance was negatively associated with unlogged forests. Host tree species, tree height, and presence of moss on branches were positively associated with the abundance of epiphytes. Despite being ecologically important, no study has thus far examined the impact of selective logging on the epiphyte assemblage in the Western Ghats. Our findings contribute to the knowledge of vascular epiphytes from South and Southeast Asia and set the stage for future research and conservation.
Introduction
Forest canopies are defined as the aggregate of tree crowns in a stand of vegetation (Parker, 1995) and are a dynamic, functional interface between terrestrial biomass and the atmosphere (Ozanne et al., 2003; Nakamura et al., 2017). The canopies support diverse organisms ranging from insects to plants (Erwin, 1991; Nadkarni, 1994; Lowman and Schowalter, 2012). The presence of this rich diversity has been attributed to the structural complexity, availability of microhabitats, and resources within the canopy (reviewed in Ozanne et al., 2003). The loss or alteration to forests and the canopy is bound to profoundly influence aspects such as nutrient fluxes, carbon sequestration, plant water relations, and biodiversity support (Ozanne et al., 2003; Nadkarni et al., 2011; Lowman, 2020). Although globally acknowledged to be an important habitat, our knowledge and understanding of the canopies has only begun to gradually expand over the past four decades (Lowman, 2020).
Forest canopies serve as an important habitat for plants that germinate and root non-parasitically on other plants at all stages of life, termed epiphytes (Benzing, 2004; Zotz, 2016). Vascular epiphytes are diverse, comprising over 31,100 species distributed globally and accounting for nearly 10% of all extant plants (Benzing, 2004; Nakamura et al., 2017; Zotz et al., 2021). Although distributed globally, the diversity of vascular epiphytes peaks in the humid subtropical and tropical regions (Zotz and Hietz, 2001; Wolf and Flamenco-S, 2003; Zotz et al., 2021). Vascular epiphytes are vertically stratified on trees (Benzing, 1995) and occupy a three-dimensional space within the canopy, resulting in varying distribution patterns when observed at different ecological scales (Mendieta-Leiva and Zotz, 2015) such as individual trees (e.g., Freiberg, 1996), vertically on trees (e.g., Petter et al., 2015), across forest stands (e.g., van Leerdam et al., 1990; Nieder et al., 2000; van Dunné, 2002; Wolf and Flamenco-S, 2003; Alvarenga et al., 2009), and elevation gradients (e.g., Cardelús et al., 2005; Ding et al., 2016). Irrespective of their distribution, vascular epiphytes are vital to maintaining several canopy-atmosphere interactions (reviewed in Lowman and Schowalter, 2012) including nutrient flux regulation; temperature regimes (Scheffers et al., 2014); and plant-water relations (Van Stan and Pypker, 2015). They also enhance the structural and functional diversity of the canopy ecosystem by providing resources to a wide variety of organisms ranging from insects to mammals (Nadkarni and Matelson, 1989; Nadkarni and Longino, 1990; Nakamura et al., 2017).
Observed distribution patterns are attributed to the physiology of the epiphyte (Zotz and Hietz, 2001), microclimatic conditions (Krömer et al., 2006), and a varying degree of affinity to host tree characteristics (Wagner et al., 2015). Host trees are the fundamental unit of habitat for epiphytes, and it follows that tree size and architecture influence the diversity of epiphytes (Flores-Palacios and Garcia-Franco, 2006; Zotz and Schultz, 2008; Wolf et al., 2009; Zhao et al., 2015). Typically, large trees with greater diameter are older and have had greater time for epiphyte colonization events as well as a greater variation in light and humidity (Benzing, 2004; Burns, 2007). Furthermore, aspects of the host tree such as substrate characteristics (Kernan and Fowler, 1995), phenology (Einzmann et al., 2015), and branch throughfall (Winkler et al., 2005) may affect the patterns of epiphyte colonization and survival (Callaway et al., 2002). Disturbance from selective logging of host trees is predicted to negatively affect the vascular epiphyte assemblage (Petter et al., 2021) and indeed, studies have documented negative impacts of selective logging on epiphytes (e.g., Padmawathe et al., 2004; Wolf, 2005; Woods and DeWalt, 2013). However, there is increasing evidence indicating that selective logging where old-growth vegetation is retained can offset the negative impact and the epiphyte assemblages are similar to unlogged forests (Hietz, 2005; Hietz et al., 2006; Lõhmus and Lõhmus, 2010). As rapid deforestation continues in the tropics (Koh et al., 2004; Asner et al., 2005; Broadbent et al., 2008; Sodhi et al., 2009; Laurance, 2013; Struebig et al., 2015), it is imperative to document the impacts on diverse epiphyte assemblages.
The Western Ghats of India is one of the densely populated regions (Cincotta et al., 2000) and the natural landscapes are being threatened by increasing fragmentation and land-use change (Cincotta et al., 2000; Critical Ecosystem Partnership Fund, 2007). The Western Ghats of India and Sri Lanka are a global biodiversity hotspot (Myers et al., 2000) and while much of the landscape has been converted to different land uses, a large proportion of the natural landscape has been formally protected (Das et al., 2006). The vascular epiphyte assemblage in the Western Ghats has not been systematically studied and existing knowledge is from numerous regional checklists (e.g., Ganesan and Livingstone, 2000; Annaselvam and Parthasarathy, 2001; Mathew and George, 2015; Sebastian et al., 2021). The epiphyte diversity is likely to be underestimated because the forest canopy has largely been ignored. Furthermore, there is no systematic investigation of the impacts associated with activities such as logging of trees or forest stands, on vascular epiphyte assemblages, especially within the forest canopy of the Western Ghats.
We aimed to understand how disturbance to forests in the form of selective logging affects the vascular epiphyte assemblage. We achieved this by systematically documenting the epiphyte abundance and diversity in the forest canopy of wet-evergreen forests of the southern Western Ghats. A part of the wet-evergreen forests of the Kalakad Mundanthurai Tiger Reserve (KMTR), Tamil Nadu was selectively logged ∼40 years ago and this has altered the vegetation, impacting the regeneration process (Ganesan, 2000; Ganesan and Davidar, 2003; Nerlekar et al., 2019). Furthermore, long-term ecological research in KMTR has also documented the persisting effects of selective logging on taxonomic groups such as amphibians (Seshadri, 2014), birds (Ramachandran and Ganesh, 2012), butterflies (Devy and Davidar, 2001), lizards (Ishwar et al., 2003), and small carnivores (Mudappa et al., 2007). So far, 55 species of epiphytes have been documented from KMTR with a subset of them being restricted to the forest canopy (Ganesan and Livingstone, 2000).
Here, we (1) document the richness, diversity, and abundance of vascular epiphyte assemblages in unlogged and selectively logged forests. Given that selective logging of trees alters the substrate available for epiphytes, we hypothesized selectively logged forests to be lower in epiphyte species richness, diversity, and abundance; (2) compare the niche width and overlap of epiphytes, using the host tree as a resource, between the unlogged and selectively logged forests. During the selective logging activities, specific host tree species were logged, and we hypothesized that the loss of tree resources would result in narrower niches and greater extent of overlaps among epiphytes in the selectively logged forest; and (3) identify characteristics of the host tree as well as the microhabitat that are associated with the abundance of vascular epiphytes. We expected the abundance to be negatively associated with the selectively logged forest. Our novel findings serve as the baseline information to investigate the associations of epiphytes with their host trees and habitats in other parts of India. Our findings are at the interface of understanding forest canopy and disturbance ecology with broader implications for conserving vascular epiphytes in South and Southeast Asia.
Materials and Methods
Study Area
We sampled trees in the wet-evergreen forests of Kalakad Mundanthurai Tiger Reserve (KMTR, 8°25′ to 8°53′ N and 77°10′ to 77°35′ E, 900 km2) located within the Agasthyamalai Biosphere Reserve in the southern Western Ghats (Figure 1). The study area is located between 1,300 and 1,400 m asl and receives a mean annual rainfall of 3,000–3,500 mm, from the Northeast and the Southwest monsoon with a pronounced dry season between January to May (Ganesh et al., 1996). Trees in two contiguous sites, Kakachi (unlogged) and Upper Kodayar (selectively logged), were sampled in an area of ca. 4 km2 each and are located within a north–south oriented saddle among hill ranges of KMTR. The unlogged forest comprises Cullenia-Palaquium-Aglaia vegetation type and the selectively logged forest comprises Cullenia-Calophyllum-Aglaia type (Ganesh et al., 1996; Ganesan, 2000). Trees such as Cullenia exarillata, Palaquium ellipticum, Myristica dactyloides, and Calophyllum austroindicum were logged in Upper Kodayar until the year 1988 for timber, supplied to the matchstick industry. The effects of selective logging on the tree vegetation were apparent even after 20 years after logging ceased (Ganesan and Davidar, 2003) and continue to linger on recruitment of trees even after 40 years (Nerlekar et al., 2019). The trees in unlogged and selectively logged forests grow between 30 and 35 m in height and have comparable average mean annual temperature (unlogged = 18.1°C vs. Selectively logged = 18.7°C) and relative humidity (unlogged = 71.7% vs. selectively logged = 76.7%). However, the light irradiance levels are nearly 15 times greater in the selectively logged forest (667 lux/m2) than unlogged forest (44.5 lux/m2; Ganesh and Tamizalagan, 2012).
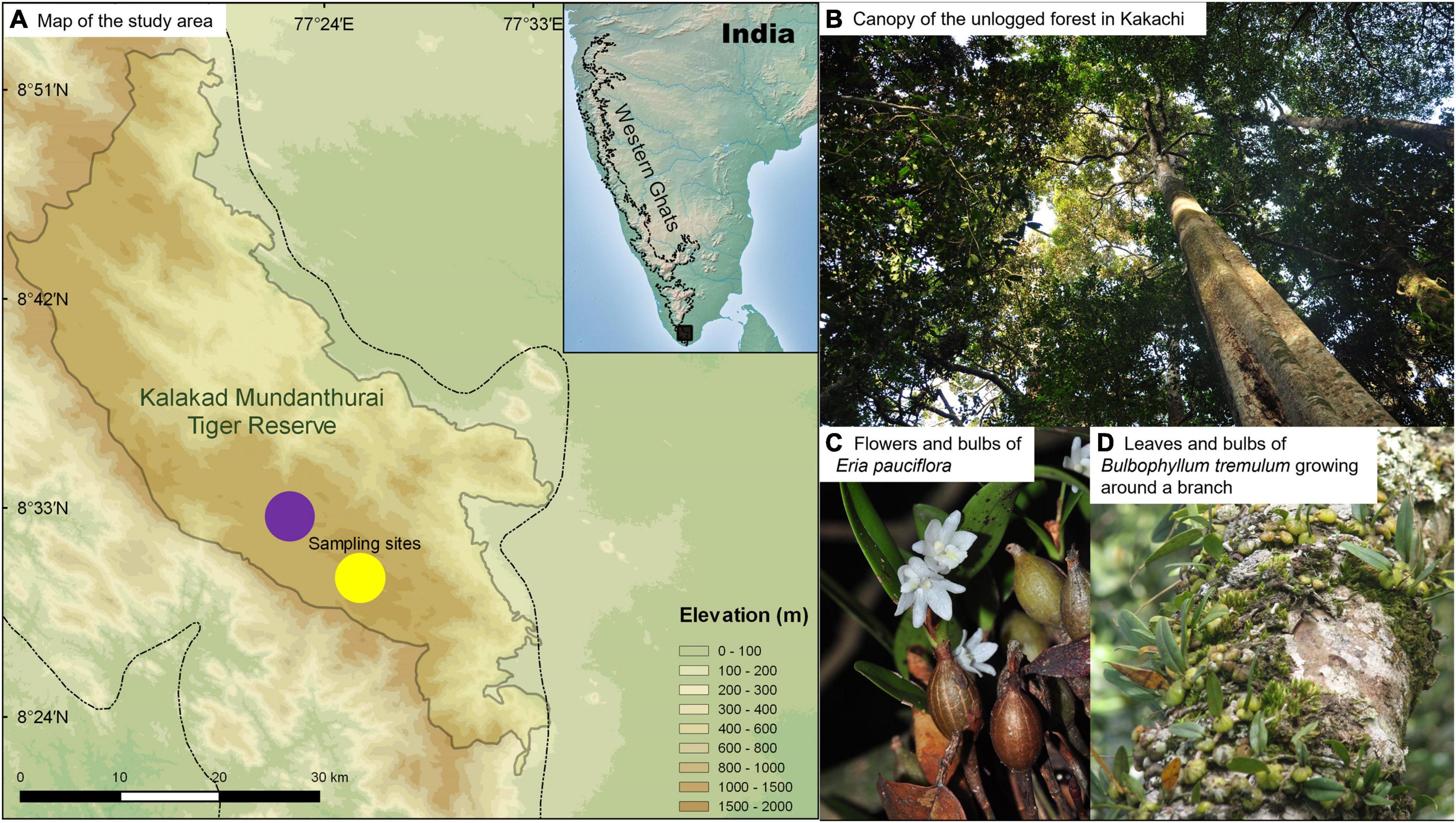
Figure 1. (A) Map showing the study area. The dotted line indicates the Western Ghats. Circles represent the two sampling sites, Kakachi (purple, unlogged) and Kodayar (gold, selectively logged). (B) The forest canopy is seen from the ground in Kakachi. (C) Flowers and bulbs of Eria pauciflora. (D) Leaves and bulbs of Bulbophyllum tremulum growing around a branch of a Cullenia exarillata tree.
Study Design
The vascular epiphyte assemblage was our study unit, the host tree, our sampling unit at the scale of selectively logged and unlogged forest (sensu Mendieta-Leiva and Zotz, 2015). We chose 100 trees each in the unlogged and selectively logged forests using a stratified random sampling approach. In a stratified search, trees were selected by two persons walking off from existing trails in the forest and scanning branches for epiphytes using binoculars (Bushnell® 8X40). Any tree >25 cm in diameter at breast height and having epiphytes on more than five branches was randomly chosen and termed the “focal” tree and the nearest four trees irrespective of epiphyte presence were chosen as “satellite” trees. Five such trees formed a cluster, resulting in 20 clusters in the unlogged and selectively logged forests, respectively. Clusters were 50–100 m apart and this design was used for analyzing neighborhood effects on the vascular epiphyte assemblage, as part of another study. To account for the habitat heterogeneity, 30 trees (6 clusters) were within 10 m of a stream and were considered to represent the riparian microhabitat type and the remaining 70 (14 clusters) trees were beyond 10 m and represented the non-riparian microhabitat type.
Trees were accessed using a modified single rope technique (Perry, 1978) by at least two persons and data collection was spread over 3 years from April 2008 to January 2010. Each spatially explicit epiphyte, including juveniles, was considered as an individual (Sanford, 1969). The number of epiphyte species, host tree species, microhabitat type (riparian or non-riparian), and geographic coordinates were recorded for each host tree. The following parameters were quantified for each sampling unit: tree height (m): from the base of the tree to the topmost part of the crown using a 50 m measuring tape; crown diameter (m): the extent of the tree crown, averaged from measurements of the distance from tree trunk to the edge of the crown in eight directions (N, NE, E, SE, S, SW, W, and NW); diameter of the tree: diameter of the trunk (DBH), measured 1.2 m above ground; association of moss with epiphyte occurrence: the presence or absence of moss or lichen on the tree branch at the site of epiphyte growth. Epiphyte species were ascertained in the field based on morphology using keys (Fischer, 1928; Sathish Kumar and Manilal, 1994).
Data Analysis
The number of epiphyte species was used as species richness. Shannon (H′) index was used as a measure of diversity and followed up with a Diversity t-test (Hutcheson, 1970) to compare diversity between unlogged and selectively logged forests. Rank abundance curves of epiphytes were compared using a log-series distribution (Krebs, 1989) and followed up with a chi2 goodness of fit test (Hammer et al., 2001). The evenness of the vascular epiphyte assemblage was computed using Simpson’s index (Magurran, 2013). The relative abundance of epiphytes occurring thrice or more; abundance of epiphyte species on host trees; and host tree characteristics (tree height, average crown diameter, and average branch girth of the host tree) followed a non-normal distribution, and their ranks were compared using a Mann–Whitney U test. All the aforementioned analyses were performed in PAST® (Hammer et al., 2001). The occurrence and abundance of epiphytes on at least three host tree species was used to compute niche width using Levin’s index and niche overlap was measured using Pianka’s Index (Pianka, 1974) using the “spa” package (Zhang, 2016) in R ver. 4.0.5 (R Core Team, 2021). Levin’s index is used to compute niche width for species occurring in different resources (Feinsinger et al., 1981) and Pianka’s index uses a resource utilization matrix to compute pair-wise overlap values (Pianka, 1973, 1974). Levin’s index values for those epiphyte species occurring more than thrice and common to both the unlogged as well as the selectively logged forest were compared using a Wilcoxon Rank Test (Hammer et al., 2001).
A generalized linear mixed model was used to examine the association of epiphyte abundance with host tree and habitat characteristics. This class of analysis was chosen because the inherent structure in our study design of 40 clusters and mixed models allow us to include a random effect (Bolker et al., 2009). The continuous variables were chosen based on variance-inflation factor (VIF) scores and variables scoring < 3 were retained (Zuur et al., 2009). Although DBH, crown diameter, and tree height scored < 3 VIF values, we found them to be correlated, and thus, only tree height was retained for the model. The abundance of epiphytes was a dependent variable in the generalized linear mixed-effects model. Fixed effects included the four categorical variables (habitat type: selectively logged vs. unlogged; association of moss with epiphyte occurrence: moss present vs. absent; microhabitat: riparian vs. non-riparian; host tree family: 16 families (Achariaceae; Anacardiaceae; Calophyllaceae; Clusiaceae; Ebenaceae; Elaeocarpaceae; Fabaceae; Lauraceae; Malvaceae; Meliaceae; Moraceae; Myristicaceae; Rubiaceae; Salicaceae; Sapotaceae; Unidentified) which were factorized and tree height was scaled before model fitting. The host tree family was used as a surrogate because the model failed to converge upon using the 22 host tree species as a fixed effect. Epiphytic abundance being a “count” variable (meaning it was never negative), a Poisson error function was used. The function “glmer” in the “lme4” package ver.1.1-27 (Bates et al., 2015) was used to perform the mixed-effects analysis in R ver. 4.0.5 (R Core Team, 2021).
Results
Overview of the Vascular Epiphyte Assemblage
We found 2,129 individuals of vascular epiphytes (mean epiphyte abundance ± SD = 10.6 ± 17.4 indiv./tree; range = 0–115) belonging to 20 species, 13 genera, and 6 families (including two unidentified species, genera, and family) on 173 host trees of 22 species, 22 genera, and 17 families (including two unidentified species, genera, and family). Members of Orchidaceae comprised 85% of epiphyte abundance followed by Piperaceae (9.3%) and Melastomataceae (3.2%). Two other families, including one unidentified group, comprised the remaining 2.5% abundance (Table 1). The vascular epiphyte assemblage was dominated by Eria pauciflora followed by Bulbophyllum sp1 and Oberonia brunoniana. Among the 20 species, six are endemic to the Western Ghats, four have a pan-Asian distribution, three are endemic to the Western Ghats-Sri Lanka biodiversity hotspot, and one is found in India, Indochina, and Malesia. The remaining species were identified only to genera or as taxonomically distinct units and thus, their geographic distribution could not be determined. Epiphytes almost always co-occurred with moss and lichens in both unlogged and selectively logged forest (epiphyte individuals in unlogged: with moss present = 705, absent: 2; selectively logged: with moss present = 1,421, absent = 1). Additionally, we found 36 individuals of accidental epiphytes (trees and shrubs) and mistletoes, all greater in abundance and species richness in the selectively logged forest (5 species, 28 individuals) than unlogged forest (1 species and 8 individuals).
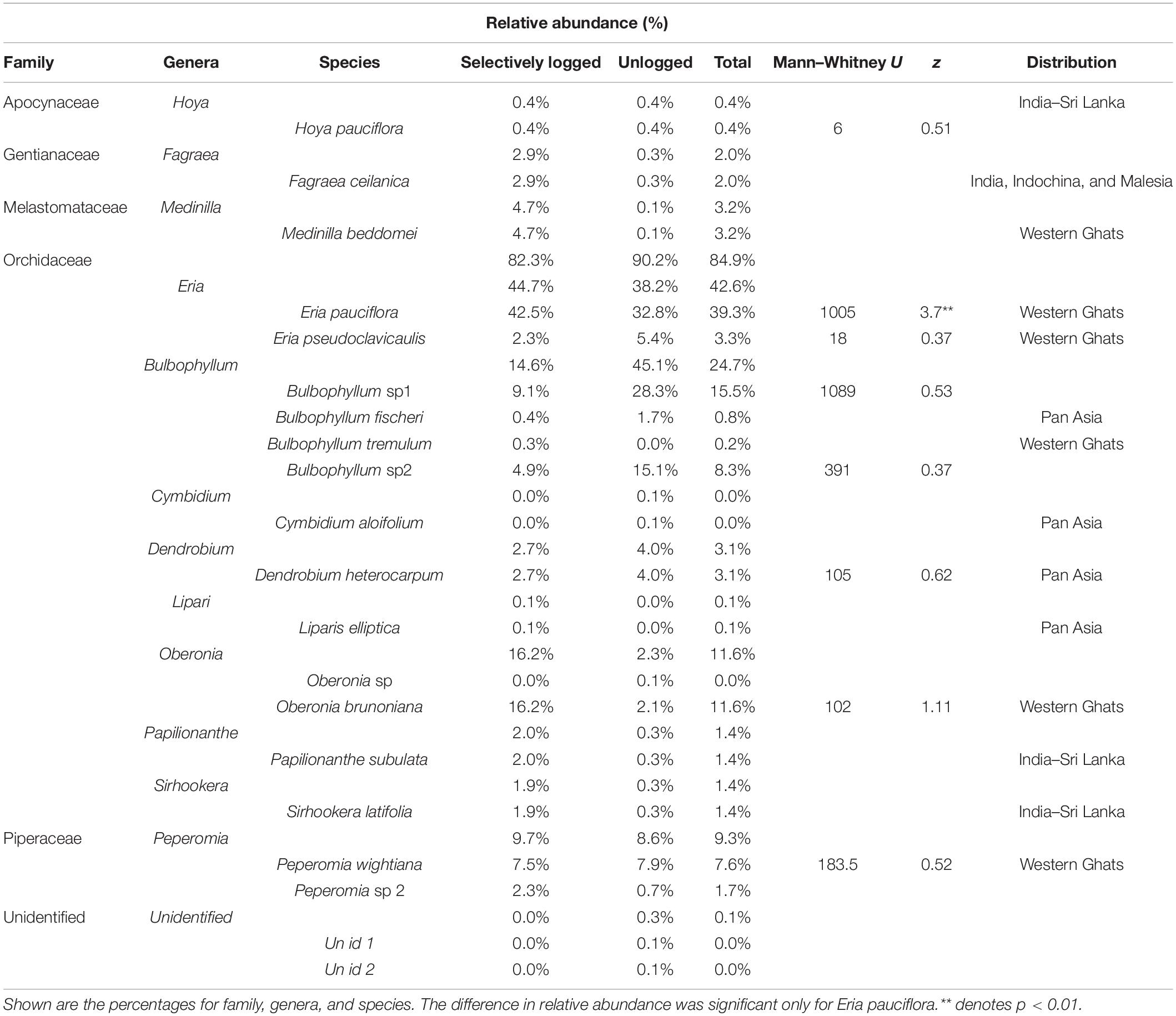
Table 1. Relative abundance of vascular epiphytes encountered in the unlogged and selectively logged forests of the Kalakad Mundanthurai Tiger Reserve (KMTR).
Species richness was similar between the selectively logged (N = 16) and the unlogged forests (N = 18). The composition of the vascular epiphyte assemblage was significantly more diverse in the selectively logged forest (Shannon H’ = 1.95) compared to the unlogged forest (Shannon H’ = 1.8; t = 3.14, df = 1559.7). The vascular epiphyte assemblage followed a log series distribution in both forests (selectively logged vs. unlogged; α = 2.5 vs. 3.36; x = 0.99 vs. 0.99; chi2 = 339.6 vs. 46.49; p < 0.05; Figure 2). The vascular epiphyte assemblage was more even in the selectively logged (evenness index = 0.44; range = 0.41–0.46) compared to the unlogged forests (evenness index = 0.33, range = 0.32–0.41) with 14 species being common to both. Two species were unique to the selectively logged and four were unique to the unlogged forest. Overall, the relative abundance of epiphytes was significantly lower in the unlogged forest (707 indiv.) compared to the selectively logged forest (1,422 indiv.; Mann–Whitney U: 2903, z = 2.55, p = 0.009). Among the epiphyte species encountered, E. pauciflora and Bulbophyllum sp1 were the two most abundant species in both the selectively logged and unlogged forest. While O. brunoniana was the third most abundant in selectively logged forest, Bulbophyllum sp2 was the third most abundant species in the unlogged forest (Figure 3). Only eight epiphyte species were encountered more than thrice each in selectively logged and unlogged forest and among them, the difference in abundance of E. pauciflora was statistically significant (Table 1).
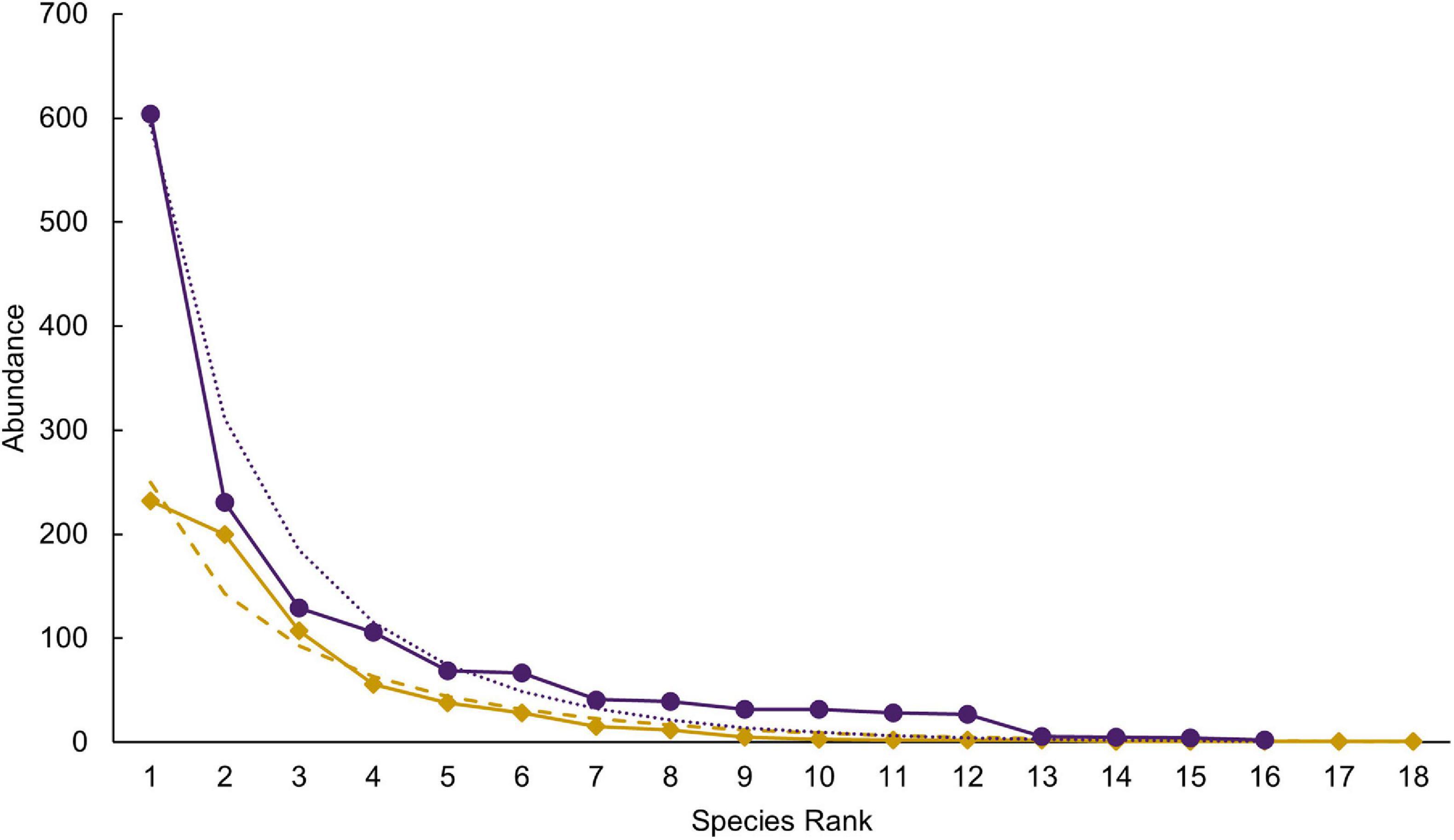
Figure 2. The epiphyte rank abundance followed a log-series distribution in both the unlogged (dots and solid line in purple) and the selectively logged forest (diamond and solid line in gold). Species abundances are ranked with model estimates shown in purple dots (unlogged) and gold dashes (selectively logged).
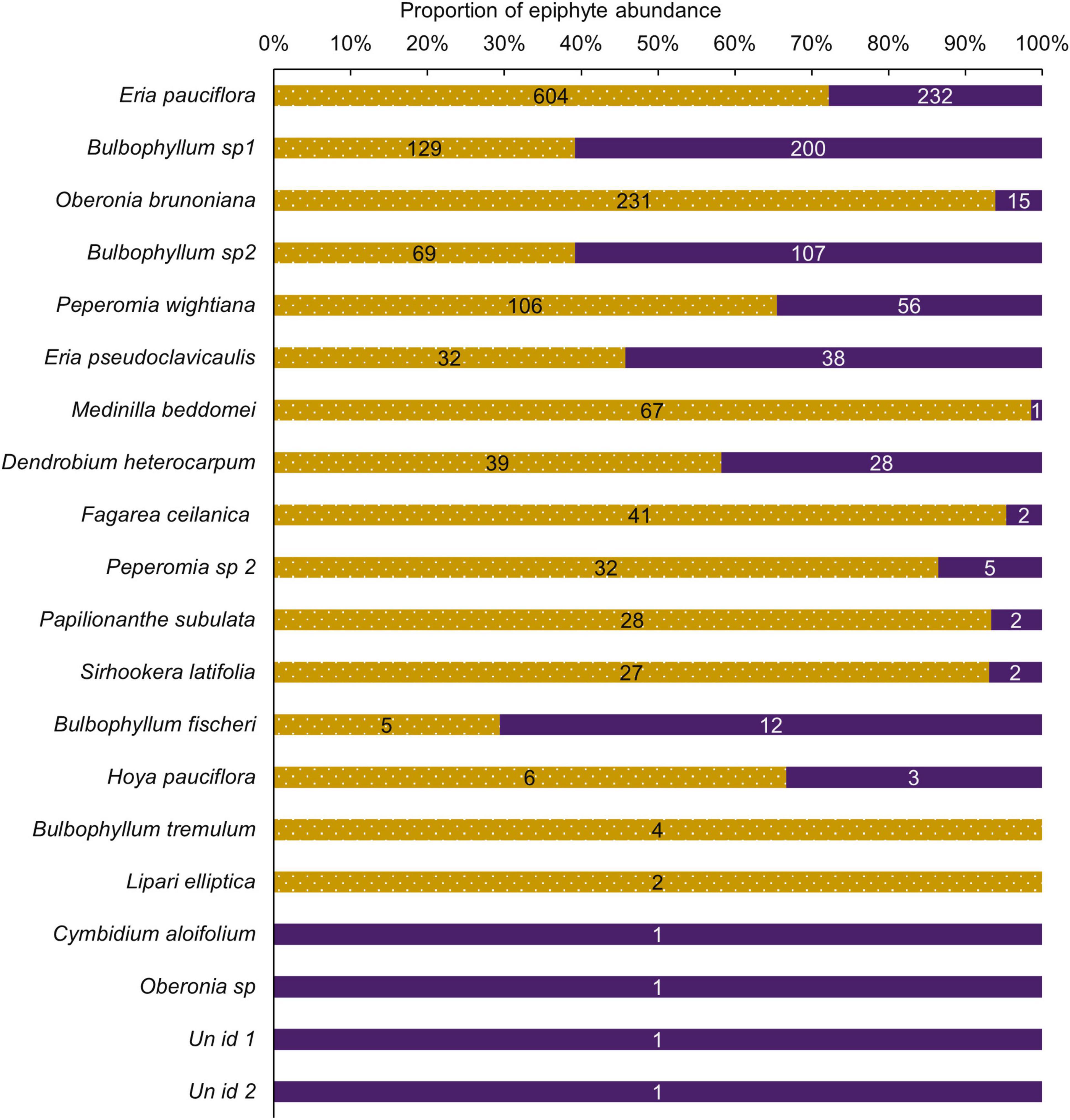
Figure 3. Proportions of epiphyte species composition differed between unlogged and selective logged forests. Relative abundance of epiphytes in the unlogged (purple) and the selectively logged forest (dotted gold). The percentage of the total abundance is depicted. Actual relative abundance values are presented on bars.
Host Tree Composition
Epiphytes were encountered on 22 host tree species belonging to 22 genera, and 17 families including two unidentified tree species. Four tree species that hosted epiphytes were common to both the selectively logged and unlogged forest, 14 species were unique to the selectively logged, and four were unique to the unlogged forest. Overall, C. exarillata hosted the highest abundance of epiphytes in both selectively logged (n = 1096) and unlogged forest (n = 501) and the difference was statistically significant (Figure 4; Mann–Whitney U = 649.5, z = 3.55, p < 0.01). Among all the host tree species, C. exarillata was taller in the unlogged (26.5 ± 3.1 m, N = 49) compared to the selectively logged forests (23 ± 2.3 m, N = 46; Mann–Whitney U = 427.5, z = 5.2, p < 0.01) forest, but the crown diameter did not differ between unlogged (5.3 ± 2 m, N = 47) and selectively logged forest (5.3 ± 1.7 m, N = 45; Mann–Whitney U = 997.5, z = 0.5, p > 0.5). The differences in characteristics of other host tree species, common to both the unlogged and selectively logged forests, were not compared because of insufficient encounters (Supplementary Table 1).
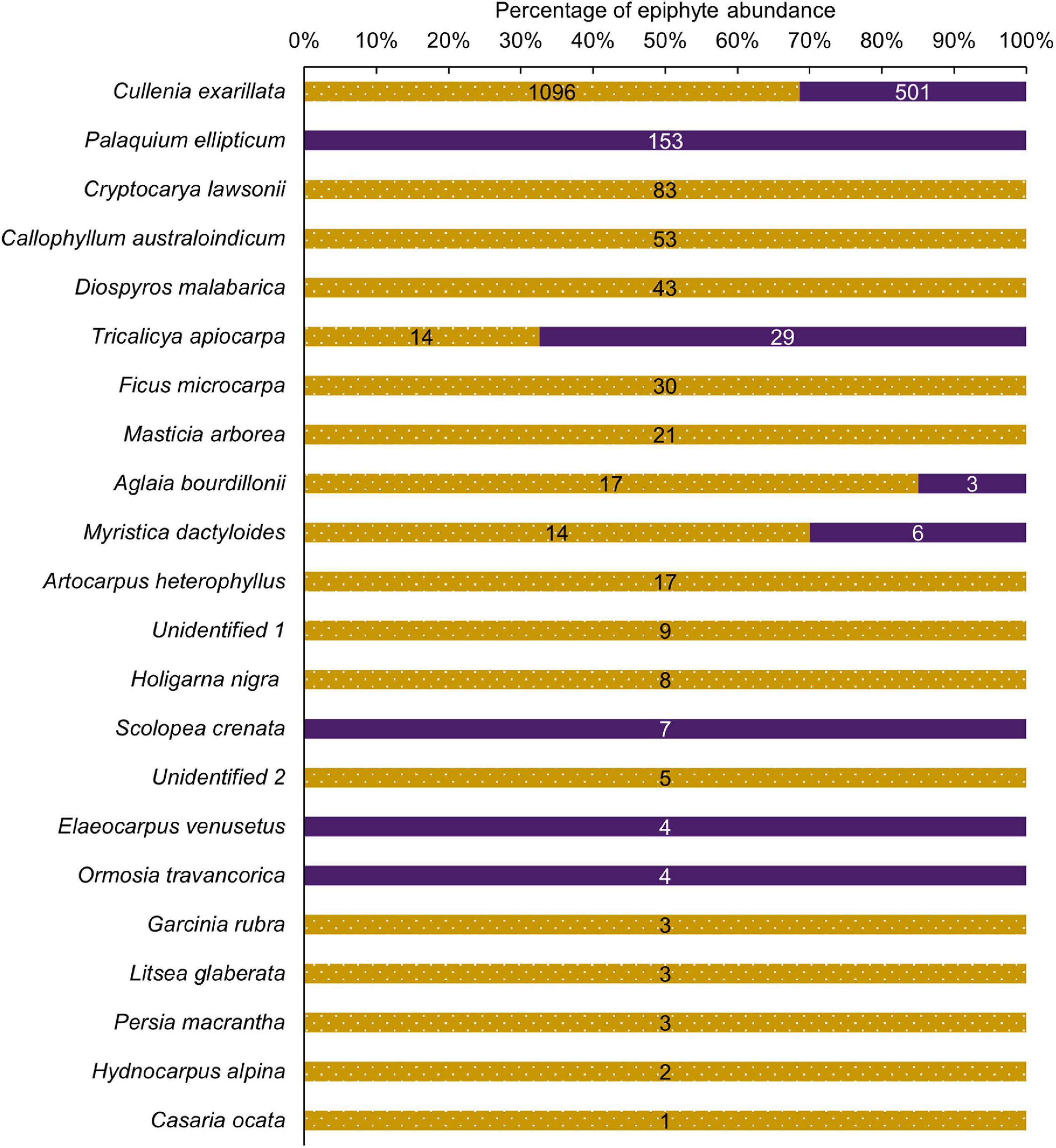
Figure 4. Relative abundance of vascular epiphytes encountered on host tree species in the unlogged (purple) and the selectively logged forest (dotted gold) forest is shown as a percentage of the total abundance. Actual relative abundance values are shown on respective bars.
Niche Width and Overlap on Host Trees
The niche widths of epiphytes occurring on three or more host tree species, and common to both selectively logged (Levin’s Index, range = 1.1–4.8) and unlogged forest (Levin’s Index, range = 1.1–2.0) were similar (Wilcoxon rank test, W = 15, z = 0.9, p > 0.05). In both the unlogged as well as the selectively logged forests, O. brunoniana had the widest niche whereas Peperomia wightiana had the narrowest niche in the unlogged forest and Eria pseudoclavicaulis had the narrowest niche in the selectively logged forest (Table 2). In the selectively logged forest, 28 species pairs overlapped by over 90% with 14 species pairs overlapping completely and the least extent of overlap was between E. pseudoclavicaulis and Papilionanthe subulata (0.40; Supplementary Table 2). In the unlogged forest, 15 species pairs overlapped by over 90%, only one species pair overlapped completely (Bulbophyllum fischeri and Bulbophyllum sp2), and the least extent of overlap was between E. pseudoclavicaulis and Hoya pauciflora (1.7; Supplementary Table 2). The greatest extent of niche overlap between species was similar between the unlogged and selectively logged forest but the lowest extent of overlap was smaller in the unlogged forest (Table 3).
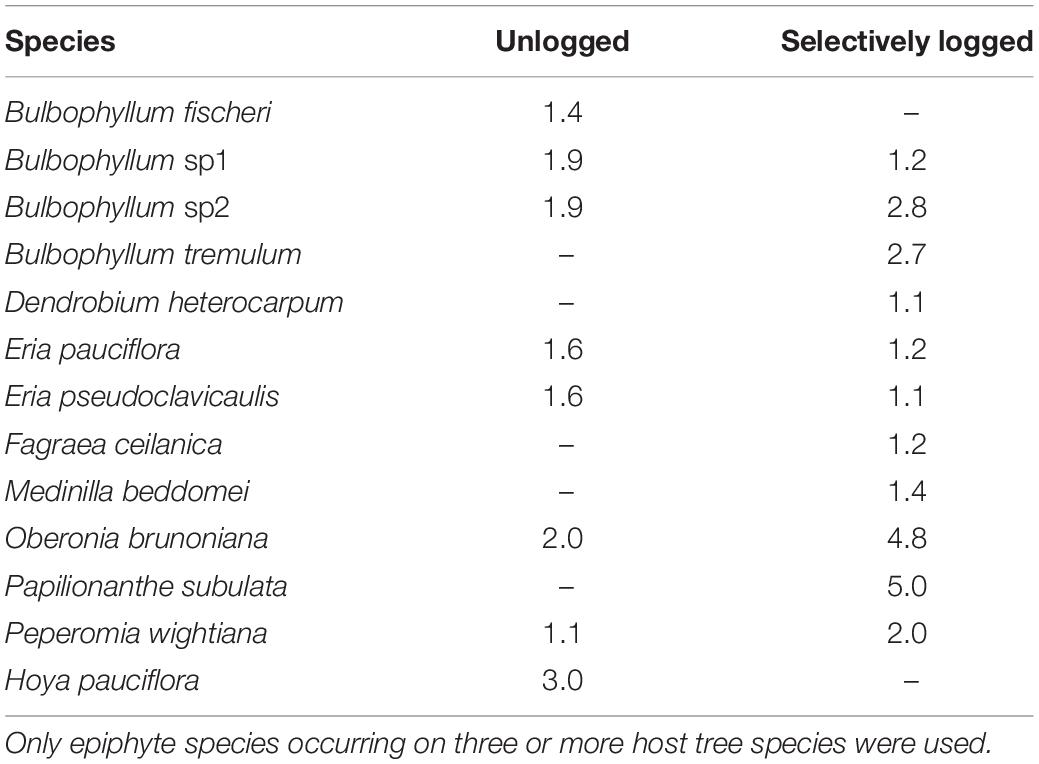
Table 2. Niche width of vascular epiphytes computed using the Levin’s Index are presented for unlogged and selectively logged forests.
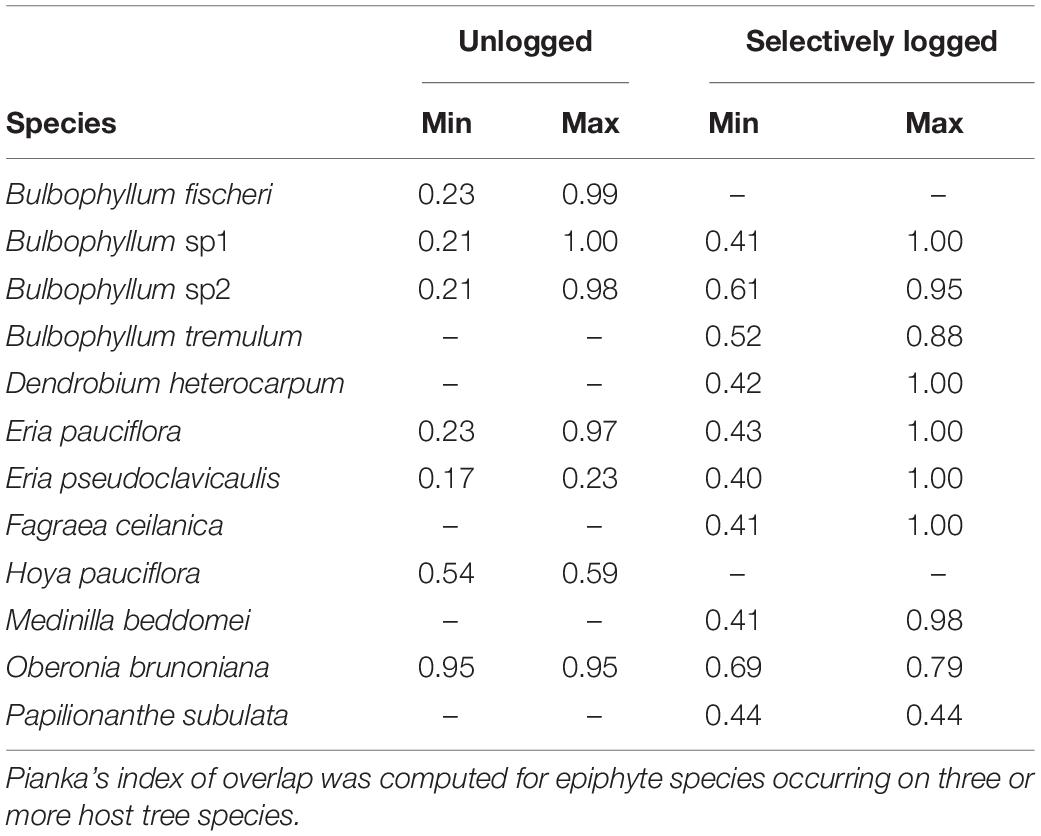
Table 3. The minimal and maximal niche overlap values of 12 vascular epiphytes encountered in the unlogged and selectively logged forests are presented.
Factors Determining the Vascular Epiphyte Abundance
The maximal additive model tested the influence of five variables as fixed effects and one random variable as a random effect on the abundance of epiphytes. Only four fixed effects had a statistically significant effect on epiphytic abundance (AIC = 2050.3, residual df = 179; cluster ID as random effect [variance ± SD]: 0.5 ± 0.7; Table 4). Epiphyte abundance was negatively associated with the unlogged forest. Epiphyte abundance was positively associated with tree height, the presence of moss, and four of the 16 host tree families (Figure 5). The association with riparian microhabitat was positive but the difference between non-riparian microhabitat was not statistically significant.
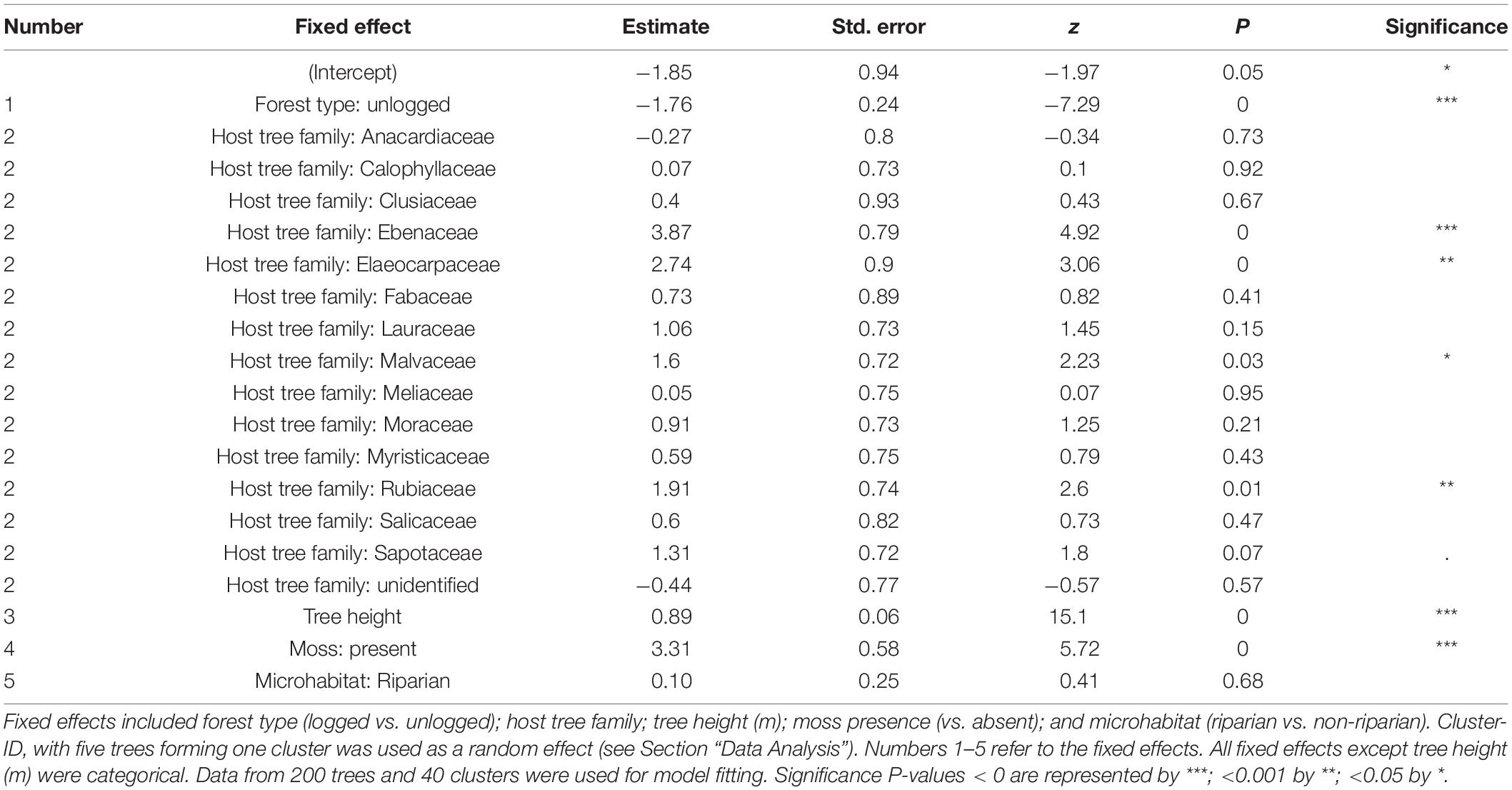
Table 4. The output of a generalized linear mixed-effects model built using the abundance of epiphytes on the individual tree as a dependent variable.
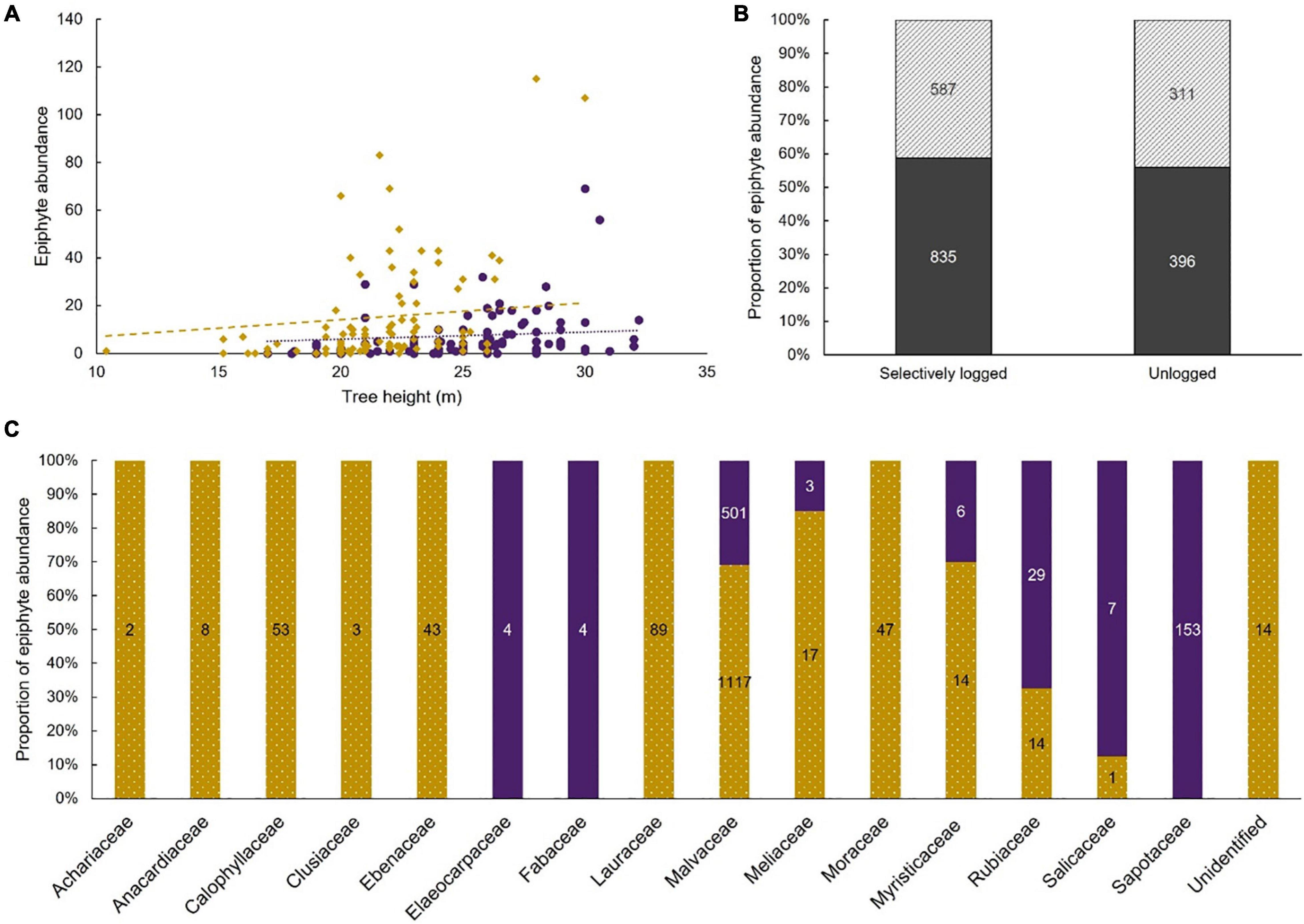
Figure 5. Epiphyte abundance was positively associated with increasing tree height (A). Associations within the unlogged forest (purple dots) and the selectively logged forest (gold diamond) are shown along with trendlines for unlogged (purple dotted) and selectively logged (gold dashed); similar proportions of epiphytes were found in the riparian (diagonal stripes) and non-riparian (solid gray) microhabitats of both the unlogged and the selectively logged forest (B). Abundance values are depicted on respective bars, and epiphyte abundance was positively associated with host tree species in the family Ebenaceae, Elaeocarpaceae, Malvaceae, and Rubiaceae. The proportion of epiphyte abundance for each host tree family in the unlogged (solid purple) and the selectively logged forest (dotted gold) are depicted (C).
Discussion
The ecology of vascular epiphytes growing in the forest canopies of tropical forests remains understudied. Access to forest canopies has been an impediment but since the 1980s methods such as the single rope technique have enabled easy access (reviewed in Lowman and Schowalter, 2012). Using the single-rope technique to access canopies, we documented the presence of a diverse assemblage of epiphytes (20 species, 2,129 individuals) growing in the forest canopy of the wet-evergreen forest in the southern Western Ghats of India. The vascular epiphyte assemblage was greater in diversity as well as relative abundance in the selectively logged forest. Epiphytes were encountered on more host tree species in the selectively logged forest but one host tree species, C. exarillata, hosted most of the epiphyte abundance in both selectively logged and unlogged forests. The niche breadth and overlap of epiphyte species, computed using the host tree as a resource, was wider and epiphyte species overlapped with each other to a greater extent in the selectively logged compared to the unlogged forest. Overall, epiphyte abundance was negatively associated with unlogged forest and positively associated with host tree height, presence of moss, and host trees belonging to four families. The location of the host tree in the riparian and non-riparian microhabitat did not have any statistically significant effect on epiphyte abundance. Taken together, our findings indicate that while the vascular epiphyte assemblage is influenced by the host tree characteristics, the effects of historical selective logging continue to persist even after 40 years since logging ceased. There are currently no other studies examining the ecological determinants of vascular epiphytes in the forest canopy in the Western Ghats, and our findings serve as a baseline for further research into the nature of epiphyte ecology in India as well as other parts of South and Southeast Asia.
The Vascular Epiphyte Assemblage
The epiphyte assemblage in both the unlogged and selectively logged forests was dominated by orchids, especially E. pauciflora, followed by Bulbophyllum spp. Both these species are drought-tolerant, and their increased abundance suggests that the epiphyte assemblage is responding to microclimate perturbations due to selective logging. Selective logging of trees is known to shift the epiphyte assemblage from a mesic to drought-tolerant species (e.g., Wolf, 2005) and epiphytes adapt to physical environmental gradients such as variation in atmospheric moisture and light availability (Benzing, 2004). Constraints of moisture availability are likely to bear a greater influence on epiphytes compared to light irradiance or nutrition (Zotz and Hietz, 2001). Although the study site receives >3,000 mm of rainfall annually, there is a pronounced dry season between January to May. Epiphytes such as E. pauciflora grow as a dense mat with bulbous, elongated stalks ending with a pair of leaves. We speculate that the dense mat likely traps canopy litter, thus accumulating canopy soil organic matter as opposed to Bulbophyllum spp. which creep along the branch, without forming a dense mat. Individuals of Bulbophyllum spp. also shed their leaves in subsequent years and only bare discoid bulbs from the plant body (Pers. Obs.). Other species such as O. brunoniana and P. wightiana do not have any vascular bulbs and yet were found in relatively high abundance in selectively logged forest. Both species appear to have moisture conserving strategies such as thick leathery strap-shaped leaves of O. brunoniana or possess thick succulent leaves in P. wightiana. The ability to tolerate drought could be critical for epiphytes to tide over xeric conditions in some periods of the year as they do not have access to soil moisture. Studying dispersal ability, the life span, and growth rates after colonization of these epiphytes would help us understand how quickly they were able to colonize the selectively logged habitat and proliferate. Such information would also help us identify epiphyte species that may be vulnerable to the impacts of climate change.
Niche Width, Overlap, and Host Bias
The dependence of epiphyte communities on a particular host has been considered as host preference or host specificity. However, Wagner et al. (2015) argue that determining specificity is a challenge given the diversity of trait associations of both the host tree as well as epiphytes. At best, any perceived association can only be termed as host bias. Host tree characteristics determine epiphyte colonization and growth (Benzing, 1995; Benzing, 2004). A decline in host tree densities will likely affect epiphytes that have specific requirements for establishment. In this study, the unlogged forest is dominated by the Cullenia-Palaquium-Aglaia series (Ganesh et al., 1996). Among the three, C. exarillata alone supported about 80% of the epiphyte abundance in both unlogged and selectively logged forests. One of the host tree species, P. ellipticum was naturally absent in the selectively logged sites and C. exarillata supported twice the number of epiphytes. The selective logging activities specifically targeted old-growth trees of large diameter and had affected the forest structure as well as regeneration of these species in the logged sites when measured 24 years after logging activities ceased (Ganesan and Davidar, 2003). A more recent examination of the forest stands indicates that the effects are still pervasive nearly 40 years after logging (Nerlekar et al., 2019). Despite the structural changes to the forest composition, the epiphyte assemblage did not appear to be drastically affected by the loss of host trees or change in host tree composition because the niche widths were statistically similar between unlogged and selectively logged forests.
The ability of C. exarillata to host a high abundance of epiphytes could be due to a combination of its relative abundance, large size, and bark features conducive to epiphyte colonization. Bark features of host trees are an important aspect that can affect the epiphyte distribution (e.g., Wyse and Burns, 2011) as is branch orientation (Mendieta-Leiva and Zotz, 2015; Wagner et al., 2015). The bark on C. exarillata tree is flaky and accumulates a large quantity of woody dust. This, along with the accumulation of canopy soil organic matter (Nadkarni, 1994), likely offers a rich nutrient source for the establishment of epiphyte communities (Zotz, 2016). Besides, the tree exhibits cauliflorous inflorescence which results in several woody knobs all along the branches. These branches after flowering/fruiting leave a rough surface which could serve as important micro-sites for deposition of the wind-dispersed epiphytic seeds. Several mammals visit the tree for feeding on the flowers (Ganesh and Devy, 2000) and they might be aiding as secondary dispersal agents of several epiphytic species as they move to other trees. In addition, the tree architecture may be conducive to intercepting wind-dispersed spores because the branches radiate horizontally from the trunk. Branches of different orientations and directions may provision diverse microclimatic gradients varying on the axes such as light, relative humidity, and the likelihood of intercepting wind-dispersed seeds. Future research will help us gain insights into the mechanisms behind the greater epiphyte abundance on C. exarillata.
We expected the epiphytes occurring in selectively logged forests to have a narrower niche width because fewer host trees would be available for colonization. We found that the niche widths of five epiphyte species common to the two forests were unaffected, meaning that the five epiphyte species used the same range of host trees or trees in both the unlogged and selectively logged forest. We found the niche among species to overlap to a greater extent in the selectively logged forest, indicating that fewer host trees had suitability for colonization and more than one species pair was sharing the host tree as a resource. Niches vary on multi-dimensional axes, and it remains to be studied if variability in other resources such as moisture or light availability resulted in the patterns we have observed. Future studies may also be able to quantify the microclimatic variability within host trees and evaluate colonization rates by quantifying seed or spore arrival on host trees in both forests. Taken together, the unaffected niche widths and greater overlaps in selectively logged forests suggest that the epiphyte assemblage shows a moderate degree of structural host bias (sensu Wagner et al., 2015).
Determinants of the Vascular Epiphyte Assemblage
Our generalized linear mixed model analysis indicated that the epiphyte abundance was positively associated with tree height, presence of moss on branches, and four host tree families; negatively associated with unlogged forest; and did not vary between riparian on non-riparian zones. Host tree features are known to profoundly influence the abundance of epiphytes (Benzing, 2004). Several studies have found the diameter of the host tree (DBH) to be correlated with tree heights as well as epiphyte abundance (e.g., Zotz and Vollrath, 2003) and more generally, tree size is correlated with epiphyte abundance (Hietz-Seifert et al., 1996; Hietz, 2005; Wolf et al., 2009; Dislich and Mantovani, 2015). Tree height or DBH is used as a proxy for tree size and we find the tree height to be similar between both unlogged and selectively logged forests and overall, older and thus larger, trees were likely to support a greater abundance of epiphytes. A similar trend has been observed in another study from Asia by Zhao et al. (2015). The only other study from India, by Padmawathe et al. (2004), found the richness and abundance of epiphytes were significantly correlated with crown diameter but not with tree height. However, they treat crown diameter and tree height as two separate variables while the two may be correlated. While we also measured the average crown diameter and DBH, we used only tree height because the two were strongly correlated.
The presence of moss also emerged as a key driver for epiphyte abundance from our models. Mosses and lichens comprise the non-vascular epiphyte community and vary within the tree based on microclimate and substrate characteristics (e.g., Holz et al., 2002). In the temperate forests, the bryophytic vegetation is early colonizers and these poikilohydric (ability to increase or decrease moisture holding capacity) flora create thick mats of dead and decaying vegetation which enables colonization by other vascular epiphyte assemblages (Nadkarni, 2000). Studies suggest that the mats of lichens and mosses may provide a substrate for colonization of vascular epiphyte either by trapping seeds or spores or buffer the moisture requirements or all three (reviewed in Zotz, 2016). The mechanisms of seed arrival and establishment of vascular epiphytes as well as associations with other plants such as mistletoe or lianas continues to be understudied, especially in the tropics. Our finding that the vascular epiphytes were almost always found with non-vascular epiphytes indicates similarities in the community ecology of vascular epiphytes in both tropical and temperate forests. We expected the epiphyte abundance to be associated with riparian habitats because moisture availability is a known physiological constraint (Zotz and Hietz, 2001) for epiphyte colonization and survival. On the contrary, we found that the presence of the host tree in the riparian zone did not support a greater number of epiphytes compared to those in the non-riparian zones, irrespective of the logging status. The strong positive association with the presence of moss and their poikilohydric nature is likely to have buffered epiphyte assemblages to a similar extent in both selectively logged and unlogged sites. However, future evaluation of the performance of epiphyte assemblage as measured by colonization or growth rates in riparian and non-riparian zones is necessary because climate change effects will likely affect the two zones differently (Seavy et al., 2009).
The tree height and presence of moss on host trees could be attributed to the host tree characteristic itself. We were unable to account for the host tree as a fixed effect in the model because of model convergence issues and therefore, we used the host tree families as a proxy, assuming that the characteristics influencing the epiphyte assemblage would be similar among species within the family. Among the 16 families used to build the model, a positive association was observed in only four families viz., Ebenaceae (1 host tree species), Elaeocarpaceae (1 host tree species), Malvaceae (2 host tree species), and Rubiaceae (1 host tree species). Examining the five host tree species belonging to the four families (Supplementary Table 1) suggests that the rough or fissured bark texture may be aiding the epiphyte abundance. Further studies could artificially modify the substrate and determine colonization rates on these host tree species to determine if bark features are indeed an important driver for the rich abundance of epiphytes.
The status of the forest, whether selectively logged or unlogged, was statistically significant in the model and unlogged forests were negatively associated with epiphyte abundance. This was an unexpected result, and we speculate that the old-growth vegetation in the unlogged forest, the dense, unbroken canopy, and the slower recruitment of host trees resulted in a saturated habitat for epiphyte colonization. Tree falls would create gaps in the forest canopy, facilitating the recruitment of young trees, but such gaps were uncommon (Pers. Obs.). The dense and continuous canopy could also affect the wind flow and thus, seed and spore dispersal in the unlogged forest compared to the more open, selectively logged forest. Long-term studies of air temperature, relative humidity, and light irradiation in both selectively logged and unlogged sites show a marked increase in light irradiation in the selectively logged forest (Ganesh and Tamizalagan, 2012). All these factors, in combination, may have enabled the epiphyte abundance to increase in selectively logged sites, resulting in a positive association.
Conserving Epiphyte Assemblages in the Western Ghats of India
Commercial logging of trees is a major driver of land-use change and has profound consequences on the structure and function of the ecosystem (Brown and Gurevitch, 2004; Fredericksen and Fredericksen, 2004; Padmawathe et al., 2004; Asner, 2005; Ernst et al., 2006; Peres et al., 2006; Villela et al., 2006; Broadbent et al., 2008; Burivalova et al., 2014). Such land-use modifications invariably impact vascular epiphytes as they depend on existing flora for survival. In our study site, the forests were logged about four decades ago and information about its impacts on floral diversity is limited to three reports on trees and other vegetation but not on epiphytes (Ganesan, 2000; Ganesan and Davidar, 2003; Nerlekar et al., 2019). Although few studies exist in Asia, several studies have examined the impacts of logging on the epiphyte community in the neotropics and suggest that the impacts of selective logging are fewer compared to clear felling (e.g., Wolf, 2005; Wolf et al., 2009). Studies also highlight the importance of retaining old-growth trees within selectively logged sites as an effective management strategy to conserve biodiversity (Rosenvald and Lõhmus, 2008). Parts of the Western Ghats are well protected, but a diverse range of habitats lay outside the protected areas and need to be conserved (Das et al., 2006). Wild harvest of orchids for medicine and trade (Hinsley et al., 2018) may also be an emerging threat to epiphytic orchids in India and it is necessary to document its impacts.
Conclusion
Our findings indicate the presence of distinct distribution patterns of epiphytes at the scale of forest type as well as between host trees. It appears that after 40 years since logging activities ceased, the vascular epiphyte assemblage does not match that of the unlogged forest. Although there was higher epiphytic abundance in the selectively logged forest, we caution against using our findings as evidence to support indiscriminate or selective logging because we only present a snapshot of the selectively logged forest during its succession. Despite 40 years since logging, the impacts appear to persist on vascular epiphytes as a greater abundance of drought-resistant species were found in the selectively logged forest. Our study has caveats such as the unavailability of data on moisture, light irradiance, and tree architecture for all the trees sampled. The lack of a baseline before the forest was logged or from elsewhere within the Western Ghats prevents us from making unified inferences. Nevertheless, the findings open an avenue for further research about the physiology of epiphytes, the changes to faunal diversity supported by epiphytes, and highlights the need to document patterns of epiphyte colonization on trees differing in characteristics such as architecture or bark texture. Such studies would provide novel insights, expand our understanding of the forest canopy, and help conserve them in the rapidly changing forests of South and Southeast Asia.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
SD and RG designed the study and secured funding. KS collected field data and analyzed data. RG identified species. KS wrote the manuscript with inputs from SD and RG. All authors have approved the submission.
Funding
This study was financially supported by the Department of Science and Technology, Government of India, by a grant (SR/S0/AS-01/2004) to SD and RG. KS was supported by the DST-INSPIRE faculty fellowship (DST/INSPIRE/04/2019/001782) by the Department of Science and Technology during manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
C. K. Sreedharan, PCCF and Chief Wildlife Warden, Tamil Nadu Forest department provided the necessary research permits. A. Ramkumar, Conservator of Forests and Field Director, KMTR, and The Deputy Directors and Range Forest Officers, Kodayar provided their valuable suggestions and support. Tamizalagan, Johnson, Murthy, Esaki-Muttu, Mythri, Aditya, and Mathivannan provided field support. The accommodation was provided by Tamil Nadu Electricity Board. Ganesh T., Jan Wolf, and Gerhard Zotz provided critical comments on early drafts of this manuscript. Vidisha M. K. and the two reviewers provided useful suggestions to the manuscript. We thank them all.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2021.727422/full#supplementary-material
Supplementary Table 1 | Tree height and canopy spread of host trees in unlogged and selectively logged forests.
References
Alvarenga, L. D. P., Pôrto, K. C., and de Oliveira, J. R. P. M. (2009). Habitat loss effects on spatial distribution of non-vascular epiphytes in a Brazilian Atlantic forest. Biodivers. Conserv. 19, 619–635. doi: 10.1007/s10531-009-9723-2
Annaselvam, J., and Parthasarathy, N. (2001). Diversity and distribution of herbaceous vascular epiphytes in a tropical evergreen forest at Varagalaiar, Western Ghats, India. Biodivers. Conserv. 10, 317–329. doi: 10.1023/A:1016670621331
Asner, G. P. (2005). Selective logging in the Brazilian amazon. Science 310, 480–482. doi: 10.1126/science.1118051
Asner, G. P., Knapp, D. E., Broadbent, E. N., Oliveira, P. J., Keller, M., and Silva, J. N. (2005). Selective logging in the Brazilian amazon. Science 310, 480–482.
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Benzing, D. H. (2004). “Vascular epiphytes,” in Forest canopies, eds M. D. Lowman and H. B. Rinker (San Diego, CA: Academic Press), 175–211. doi: 10.1016/B978-012457553-0/50014-9
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
Broadbent, E., Asner, G., Keller, M., Knapp, D., Oliveira, P., and Silva, J. (2008). Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian amazon. Biol. Conserv. 141, 1745–1757. doi: 10.1016/j.biocon.2008.04.024
Brown, K. A., and Gurevitch, J. (2004). Long-term impacts of logging on forest diversity in Madagascar. Proc. Natl. Acad. Sci. U.S.A. 101, 6045–6049. doi: 10.1073/pnas.0401456101
Burivalova, Z., Sekercioglu, C. H., and Koh, L. P. (2014). Thresholds of logging intensity to maintain tropical forest biodiversity. Curr. Biol. 24, 1893–1898. doi: 10.1016/j.cub.2014.06.065
Burns, K. C. (2007). Network properties of an epiphyte metacommunity. J. Ecol. 95, 1142–1151. doi: 10.1111/j.1365-2745.2007.01267.x
Callaway, R. M., Reinhart, K. O., Moore, G. W., Moore, D. J., and Pennings, S. C. (2002). Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia 132, 221–230. doi: 10.1007/s00442-002-0943-3
Cardelús, C. L., Colwell, R. K., and Watkins, J. E. Jr (2005). Vascular epiphyte distribution patterns: explaining the mid-elevation richness peak. J. Ecol. 96, 144–156. doi: 10.1111/j.1365-2745.2005.01052.x
Cincotta, R. P., Wisnewski, J., and Engelman, R. (2000). Human population in the biodiversity hotspots. Nature 404, 990–992. doi: 10.1038/35010105
Critical Ecosystem Partnership Fund (2007). Ecosystem Profile Western Ghats and Sri Lanka Biodiversity Hotspot. Arlington, TX: Critical Ecosystem Partnership Fund.
Das, A., Krishnaswamy, J., Bawa, K. S., Kiran, M. C., Srinivas, V., Kumar, N. S., et al. (2006). Prioritisation of conservation areas in the Western Ghats, India. Biol. Conserv. 133, 16–31. doi: 10.1016/j.biocon.2006.05.023
Devy, M. S., and Davidar, P. (2001). Response of wet forest butterflies to selective logging in Kalakad-Mundanthurai tiger reserve: implications for conservation. Curr. Sci. 80, 400–405.
Ding, Y., Liu, G., Zang, R., Zhang, J., Lu, X., and Huang, J. (2016). Distribution of vascular epiphytes along a tropical elevational gradient: disentangling abiotic and biotic determinants. Sci. Rep. 6:19706. doi: 10.1038/srep19706
Dislich, R., and Mantovani, W. (2015). Vascular epiphyte assemblages in a Brazilian atlantic forest fragment: investigating the effect of host tree features. Plant Ecol. 217, 1–12. doi: 10.1007/s11258-015-0553-x
Einzmann, H. J., Beyschlag, J., Hofhansl, F., Wanek, W., and Zotz, G. (2015). Host tree phenology affects vascular epiphytes at the physiological, demographic and community level. AoB Plants 7:lu073. doi: 10.1093/aobpla/plu073
Ernst, R., Linsenmair, K. E., and Rödel, M.-O. (2006). Diversity erosion beyond the species level: dramatic loss of functional diversity after selective logging in two tropical amphibian communities. Biol. Conserv. 133, 143–155. doi: 10.1016/j.biocon.2006.05.028
Erwin, T. L. (1991). How many species are there?: revisited. Conserv. Biol. 5, 330–333. doi: 10.1111/j.1523-1739.1991.tb00145.x
Feinsinger, P., Spears, E. E., and Poole, R. W. (1981). A simple measure of niche breadth. Ecology 62, 27–32. doi: 10.2307/1936664
Fischer, C. E. C. (1928). “Orchidaceae,” in Flora of Presidency of Madras, ed. J. S. Gamble (London: West, Newman and Adlard).
Flores-Palacios, A., and Garcia-Franco, J. G. (2006). The relationship between tree size and epiphyte species richness: testing four different hypotheses. J. Biogeogr. 33, 323–330. doi: 10.1111/j.1365-2699.2005.01382.x
Fredericksen, N. J., and Fredericksen, T. S. (2004). Impacts of selective logging on amphibians in a Bolivian tropical humid forest. For. Ecol. Manag. 191, 275–282. doi: 10.1016/j.foreco.2003.12.012
Freiberg, M. (1996). Spatial distribution of vascular epiphytes on three emergent canopy trees in French Guiana. Biotropica 28, 345–355. doi: 10.2307/2389198
Ganesan, R. (2000). Tree Diversity and Regeneration in Logged Wet Evergreen Forests of the Agasthyamalai Range in South Western Ghats, India. Ph.D. thesis. Chennai: Madras Christian College.
Ganesan, R., and Davidar, P. (2003). Effect of logging on the structure and regeneration of important fruit bearing trees in a wet evergreen forest, southern Western Ghats, India. J. Trop. For. Sci. 15, 12–25.
Ganesan, R., and Livingstone, C. (2000). Checklist of orchids from a mid elevation evergreen forest at kakachi-kodayar, kalakkad mundanthurai tiger reserve, agasthyamalai, Southern Western Ghats. Zoos Print J. 16, 445–446. doi: 10.11609/JoTT.ZPJ.16.3.445-6
Ganesh, T., and Devy, S. (2000). Flower use by arboreal mammals and pollination of a rain forest tree in south western ghats, India. Selbyana 21, 60–65.
Ganesh, T., and Tamizalagan (2012). “Bright canopies and dark understories,” in Forest Canopies a Glimpse, eds M. S. Devy, T. Ganesh, and A. Tripathy (Bangalore: Ashoka Trust for Research in Ecology and the Environment), 88.
Ganesh, T., Ganesan, R., Devy, M. S., Davidar, P., and Bawa, K. S. (1996). Assessment of plant biodiversity at a mid-elevation evergreen forest of Kalakad-Mundanthurai Tiger Reserve, Western Ghats, India. Curr. Sci. 71, 379–391.
Hammer, Ø, Harper, D. A. T., and Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9.
Hietz, P. (2005). Conservation of vascular epiphyte diversity in Mexican coffee plantations. Conserv. Biol. 19, 391–399. doi: 10.1111/j.1523-1739.2005.00145.x
Hietz, P., Buchberger, G., and Winkler, M. (2006). Effect of forest disturbance on abundance and distribution of epiphytic bromeliads and orchids. Ecotropica 12, 103–112.
Hietz-Seifert, U., Hietz, P., and Guevara, S. (1996). Epiphyte vegetation and diversity on remnant trees after forest clearance in southern veracruz, Mexico. Biol. Conserv. 75, 103–111. doi: 10.1016/0006-3207(95)00071-2
Hinsley, A., de Boer, H. J., Fay, M. F., Gale, S. W., Gardiner, L. M., Gunasekara, R. S., et al. (2018). A review of the trade in orchids and its implications for conservation. Bot. J. Linn. Soc. 186, 435–455. doi: 10.1093/botlinnean/box083
Holz, I., Gradstein, S. R., Heinrichs, J., and Kappelle, M. (2002). Bryophyte diversity, microhabitat differentiation, and distribution of life forms in Costa Rican upper montane Quercus forest. Bryologist 105, 334–348. doi: 10.1639/0007-2745(2002)105[0334:BDMDAD]2.0.CO;2
Hutcheson, K. (1970). A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 29, 151–154. doi: 10.1016/0022-5193(70)90124-4
Ishwar, N. M., Chellam, R., Kumar, A., and Noon, B. R. (2003). The response of agamid lizards to rainforest fragmentation in the southern Western Ghats, India. Conserv. Soc. 1, 269–286.
Kernan, C., and Fowler, N. (1995). Differential substrate use by epiphytes in Corcovado National Park, Costa Rica: a source of guild structure. J. Ecol. 83, 65–73. doi: 10.2307/2261151
Koh, L. P., Dunn, R. R., Sodhi, N. S., Colwell, R. K., Proctor, H. C., and Smith, V. S. (2004). Species coextinctions and the biodiversity crisis. Science 305, 1632–1634. doi: 10.1126/science.1101101
Krömer, T., Kessler, M., and Gradstein, S. R. (2006). Vertical stratification of vascular epiphytes in submontane and montane forest of the Bolivian Andes: the importance of the understory. Plant Ecol. 189, 261–278. doi: 10.1007/s11258-006-9182-8
Laurance, W. F. (2013). “Emerging threats to tropical forests,” in Treetops at risk, eds M. D. Lowman, D. Soubadra, and T. Ganesh (New York, NY: Springer), 71–79. doi: 10.1007/978-1-4614-7161-5_5
Lõhmus, A., and Lõhmus, P. (2010). Epiphyte communities on the trunks of retention trees stabilise in 5years after timber harvesting, but remain threatened due to tree loss. Biol. Conserv. 143, 891–898. doi: 10.1016/j.biocon.2009.12.036
Lowman, M. (2020). Life in the treetops—An overview of forest canopy science and its future directions. Plants People Planet 3, 16–21. doi: 10.1002/ppp3.10125
Lowman, M. D., and Schowalter, T. D. (2012). Plant science in forest canopies - the first 30 years of advances and challenges (1980-2010). N. Phytol. 194, 12–27. doi: 10.1111/j.1469-8137.2012.04076.x
Mathew, J., and George, K. V. (2015). Checklist of Orchids of Kottavasal Hills in Achancoil Forests, southern Western Ghats, (Kollam, Kerala), India. J. Threat. Taxa 7, 7691–7696. doi: 10.11609/JoTT.o3859.7691-6
Mendieta-Leiva, G., and Zotz, G. (2015). A conceptual framework for the analysis of vascular epiphyte assemblages. Perspect. Plant Ecol. Evol. Syst. 17, 510–521. doi: 10.1016/j.ppees.2015.09.003
Mudappa, D., Noon, B. R., Kumar, A., and Chellam, R. (2007). Responses of small carnivores to rainforest fragmentation in the southern Western Ghats, India. Small Carniv. Conserv. 36, 18–26.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Nadkarni, N. M. (1994). Diversity of species and interactions in the upper tree canopy of forest ecosystems. Am. Zool. 34, 70–78. doi: 10.1093/icb/34.1.70
Nadkarni, N. M. (2000). Colonization of stripped branch surfaces by epiphytes in a lower montane cloud forest, Monteverde, Costa Rica. Biotropica 32, 358–363. doi: 10.1111/j.1744-7429.2000.tb00479.x
Nadkarni, N. M., and Longino, J. T. (1990). Invertebrates in canopy and ground organic matter in a neotropical montane forest, Costa Rica. Biotropica 22, 286–289. doi: 10.2307/2388539
Nadkarni, N. M., and Matelson, T. J. (1989). Bird use of epiphyte resources in neotropical trees. Condor 91, 891–907. doi: 10.2307/1368074
Nadkarni, N. M., Parker, G. G., and Lowman, M. D. (2011). Forest canopy studies as an emerging field of science. Ann. For. Sci. 68, 217–224. doi: 10.1007/s13595-011-0046-6
Nakamura, A., Kitching, R. L., Cao, M., Creedy, T. J., Fayle, T. M., Freiberg, M., et al. (2017). Forests and their canopies: achievements and horizons in canopy science. Trends Ecol. Evol. 32, 438–451. doi: 10.1016/j.tree.2017.02.020
Nerlekar, A. N., Kamath, V., Saravanan, A., and Ganesan, R. (2019). Successional dynamics of a regenerated forest in a plantation landscape in Southern India. J. Trop. Ecol. 35, 1–11. doi: 10.1017/S0266467418000445
Nieder, J., Engwald, S., Klawun, M., and Barthlott, W. (2000). Spatial distribution of vascular epiphytes (including hemiepiphytes) in a lowland Amazonian rain forest (Surumoni Crane Plot) of Southern Venezuela. Biotropica 32, 385–396. doi: 10.1111/j.1744-7429.2000.tb00485.x
Ozanne, C. M. P., Anhuf, D., Boulter, S. L., Keller, M., Kitching, R. L., Körner, C., et al. (2003). Biodiversity meets the atmosphere: a global view of forest canopies. Science 301, 183–186. doi: 10.1126/science.1084507
Padmawathe, R., Qureshi, Q., and Rawat, G. S. (2004). Effects of selective logging on vascular epiphyte diversity in a moist lowland forest of Eastern Himalaya, India. Biol. Conserv. 119, 81–92. doi: 10.1016/j.biocon.2003.10.024
Parker, G. (1995). “Structure and microclimate of forest canopies,” in Forest canopies, eds M. Lowman and N. Nadkarni (New York, NY: Academic Press), 73–106.
Peres, C. A., Barlow, J., and Laurance, W. F. (2006). Detecting anthropogenic disturbance in tropical forests. Trends Ecol. Evol. 21, 227–229. doi: 10.1016/j.tree.2006.03.007
Perry, D. R. (1978). A method of access into the crowns of emergent and canopy trees. Biotropica 10, 155–157. doi: 10.2307/2388019
Petter, G., Wagner, K., Wanek, W., Sánchez Delgado, E. J., Zotz, G., Cabral, J. S., et al. (2015). Functional leaf traits of vascular epiphytes: vertical trends within the forest, intra- and interspecific trait variability, and taxonomic signals. Funct. Ecol. 30, 188–198. doi: 10.1111/1365-2435.12490
Petter, G., Zotz, G., Kreft, H., and Cabral, J. S. (2021). Agent-based modeling of the effects of forest dynamics, selective logging, and fragment size on epiphyte communities. Ecol. Evol. 11, 2937–2951. doi: 10.1002/ece3.7255
Pianka, E. R. (1973). The structure of lizard communities. Annu. Rev. Ecol. Syst. 4, 53–74. doi: 10.1146/annurev.es.04.110173.000413
Pianka, E. R. (1974). Niche overlap and diffuse competition. Proc. Natl. Acad. Sci. U.S.A. 71, 2141–2145. doi: 10.1073/pnas.71.5.2141
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ramachandran, V., and Ganesh, T. (2012). Habitat structure and its effects on bird assemblages in the kalakad-mundanthurai tiger reserve (KMTR), India. J. Bombay Nat. Hist. Soc. 109, 87–95.
Rosenvald, R., and Lõhmus, A. (2008). For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. For. Ecol. Manag. 255, 1–15. doi: 10.1016/j.foreco.2007.09.016
Sanford, W. W. (1969). The distribution of epiphytic orchids in Nigeria in relation to each other and to geographic location and climate, type of vegetation and tree species. Biol. J. Linn. Soc. 1, 247–285. doi: 10.1111/j.1095-8312.1969.tb00120.x
Sathish Kumar, C., and Manilal, K. S. (1994). A Catalogue of Indian Orchids. Dehra Dun: Bishen Singh Mahendra Pal Singh.
Scheffers, B. R., Evans, T. A., Williams, S. E., and Edwards, D. P. (2014). Microhabitats in the tropics buffer temperature in a globally coherent manner. Biol. Lett. 10:20140819. doi: 10.1098/rsbl.2014.0819
Seavy, N. E., Gardali, T., Golet, G. H., Griggs, F. T., Howell, C. A., Kelsey, R., et al. (2009). Why climate change makes riparian restoration more important than ever: recommendations for practice and research. Ecol. Restor. 27, 330–338. doi: 10.3368/er.27.3.330
Sebastian, J., Kathiresan, D., and Kuriakose, G. (2021). Species diversity and abundance patterns of epiphytic orchids in Aralam Wildlife Sanctuary in Kerala, India. J. Threat. Taxa 13, 19060–19069. doi: 10.11609/jott.4852.13.8.19060-19069
Seshadri, K. S. (2014). Effects of historical selective logging on anuran communities in a wet evergreen forest, South India. Biotropica 46, 615–623. doi: 10.1111/btp.12141
Sodhi, N. S., Posa, M. R. C., Lee, T. M., Bickford, D., Koh, L. P., and Brook, B. W. (2009). The state and conservation of Southeast Asian biodiversity. Biodiv. Conserv. 19, 317–328. doi: 10.1007/s10531-009-9607-5
Struebig, M. J., Wilting, A., Gaveau, D. L., Meijaard, E., Smith, R. J., Borneo Mammal, et al. (2015). Targeted conservation to safeguard a biodiversity hotspot from climate and land-cover change. Curr. Biol. 25, 372–378. doi: 10.1016/j.cub.2014.11.067
van Dunné, H. J. F. (2002). Effects of the spatial distribution of trees, conspecific epiphytes and geomorphology on the distribution of epiphytic bromeliads in a secondary montane forest (Cordillera Central, Colombia). J. Trop. Ecol. 18, 193–213. doi: 10.1017/S0266467402002134
van Leerdam, A., Zagt, R. J., and Veneklaas, E. J. (1990). The distribution of epiphyte growth-forms in the Canopy of a Colombian Cloud-Forest. Vegetatio 87, 59–71. doi: 10.1007/BF00045656
Van Stan, J. T. II, and Pypker, T. G. (2015). A review and evaluation of forest canopy epiphyte roles in the partitioning and chemical alteration of precipitation. Sci. Total Environ. 536, 813–824. doi: 10.1016/j.scitotenv.2015.07.134
Villela, D. M., Nascimento, M. T., Aragao, L. E. O. C., and da Gama, D. M. (2006). Effect of selective logging on forest structure and nutrient cycling in a seasonally dry Brazilian Atlantic forest. J. Biogeogr. 33, 506–516. doi: 10.1111/j.1365-2699.2005.01453.x
Wagner, K., Mendieta-Leiva, G., and Zotz, G. (2015). Host specificity in vascular epiphytes: a review of methodology, empirical evidence and potential mechanisms. AoB Plants 7:lu092. doi: 10.1093/aobpla/plu092
Winkler, M., Hulber, K., and Hietz, P. (2005). Effect of canopy position on germination and seedling survival of epiphytic bromeliads in a Mexican humid montane forest. Ann. Bot. 95, 1039–1047. doi: 10.1093/aob/mci115
Wolf, J. H. D. (2005). The response of epiphytes to anthropogenic disturbance of pine-oak forests in the highlands of Chiapas, Mexico. For. Ecol. Manag. 212, 376–393. doi: 10.1016/j.foreco.2005.03.027
Wolf, J. H. D., and Flamenco-S, A. (2003). Patterns in species richness and distribution of vascular epiphytes in Chiapas, Mexico. J. Biogeogr. 30, 1689–1707. doi: 10.1046/j.1365-2699.2003.00902.x
Wolf, J. H. D., Gradstein, S. R., and Nadkarni, N. M. (2009). A protocol for sampling vascular epiphyte richness and abundance. J. Trop. Ecol. 25, 107–121. doi: 10.1017/S0266467408005786
Woods, C. L., and DeWalt, S. J. (2013). The conservation value of secondary forests for vascular epiphytes in central panama. Biotropica 45, 119–127. doi: 10.1111/j.1744-7429.2012.00883.x
Wyse, S. V., and Burns, B. R. (2011). Do host bark traits influence trunk epiphyte communities? N. Z. J. Ecol. 35, 296–301.
Zhao, M., Geekiyanage, N., Xu, J., Khin, M. M., Nurdiana, D. R., Paudel, E., et al. (2015). Structure of the epiphyte community in a tropical montane forest in SW China. PLoS One 10:e0122210. doi: 10.1371/journal.pone.0122210
Zotz, G. (2016). Plants on Plants – The Biology of Vascular Epiphytes. Cham: Springer International Publishing. doi: 10.1007/978-3-319-39237-0
Zotz, G., and Hietz, P. (2001). The physiological ecology of vascular epiphytes: current knowledge, open questions. J. Exp. Bot. 52, 2067–2078. doi: 10.1093/jexbot/52.364.2067
Zotz, G., and Schultz, S. (2008). The vascular epiphytes of a lowland forest in Panama – species composition and spatial structure. Plant Ecol. 195, 131–141. doi: 10.1007/s11258-007-9310-0
Zotz, G., and Vollrath, B. (2003). The epiphyte vegetation of the palm Socratea exorrhiza - correlations with tree size, tree age and bryophyte cover. J. Trop. Ecol. 19, 81–90. doi: 10.1017/S0266467403003092
Zotz, G., Weigelt, P., Kessler, M., Kreft, H., and Taylor, A. (2021). EpiList 1.0: a global checklist of vascular epiphytes. Ecology 102:e03326. doi: 10.1002/ecy.3326
Keywords: canopy science, community ecology, mixed-effects model, logging, single rope technique, vascular epiphyte, conservation, Western Ghats
Citation: Seshadri KS, Ganesan R and Devy SM (2021) Persistent Effects of Historical Selective Logging on a Vascular Epiphyte Assemblage in the Forest Canopy of the Western Ghats, India. Front. For. Glob. Change 4:727422. doi: 10.3389/ffgc.2021.727422
Received: 18 June 2021; Accepted: 28 September 2021;
Published: 27 October 2021.
Edited by:
Akihiro Nakamura, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Romà Ogaya, Ecological and Forestry Applications Research Centre (CREAF), SpainHelena Einzmann, Carl von Ossietzky Universität Oldenburg, Germany
Copyright © 2021 Seshadri, Ganesan and Devy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: K. S. Seshadri, seshadriks@iisc.ac.in; Soubadra M. Devy, soubadra@atree.org
 K. S. Seshadri
K. S. Seshadri R. Ganesan2
R. Ganesan2  Soubadra M. Devy
Soubadra M. Devy